![]()
 |
Molecules with Silly |
![]()
Believe it or not, some chemists do have a sense of humour, and this page is a testament to that. Here we'll show you some real molecules that have unusual, ridiculous or downright silly names. If you know of any other potential candidates for this page, please let me know. People from all over the world have sent me so many contributions to this page, that I've now had to split it into three smaller pages.
The 3D structure files of many of these molecules can be obtained by clicking on the images. Information on what you need to view these structure files can be found here.
 Stop
Press: Due to the popularity of this site, I've now written it up as a book,
entitled 'Molecules with Silly or Unusual Names', by Paul May, published
by Imperial College Press, July 2008. It is available at all good bookstores,
price around £18. It will include all your favourite molecules from this
website, plus some extra information about them. Or you can buy it online from
World Scientific or
Amazon.
Stop
Press: Due to the popularity of this site, I've now written it up as a book,
entitled 'Molecules with Silly or Unusual Names', by Paul May, published
by Imperial College Press, July 2008. It is available at all good bookstores,
price around £18. It will include all your favourite molecules from this
website, plus some extra information about them. Or you can buy it online from
World Scientific or
Amazon.
![]()
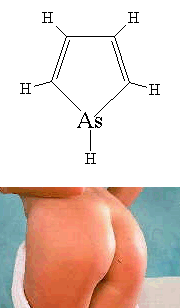
ArsoleYes, believe it or not, there is actually a molecule called
Arsole... and it's a ring! It is the arsenic equivalent of pyrrole,
and although it is rarely found in its pure form, it is occasionally seen
as a sidegroup in the form of organic arsolyls. For more
information, see the paper with probably the best title of any scientific
paper I've ever come across: "Studies on the
Chemistry of the Arsoles", G. Markl and H. Hauptmann, J. Organomet.
Chem., 248 (1983) 269. Although the class of molecules with
this general structure are called 'arsoles', the specific molecule shown
on the right is actually called 'arsenole' (not to be confused with the
London football club, Arsenal). Contrary to popular belief, new research
(see reference below) shows that arsoles are only moderately aromatic...
Incidentally US patent number US 3 412 119 by the Dow Chemical Company is
entitled 'Substituted Stannoles, Phospholes, Arsoles, and Stiboles' - I
didn't know there was a substitute for an arsole... Thanks to Neil Brookes, Nicholas Welham, Andy Shipway, Lloyd Evans, Peter Sims, John Perkins and Bob Buntrock for some of the info and details about these molecules. This article inspired Mikael Johansson from Helsinki University to do a scientific study into the aromaticity of arsoles, which has been published: Letts. Org. Chem. 2 (2005) 469. Another intriguing reference supplied by Patrick Wallace is: G. Märkl and H. Hauptmann, "Unusual Substitution in an Arsole Ring", Angew. Chem. Int. Ed. . 11, (1972) 441. Thanks also to Thomas Jeanmaire and Alan Parker for the info and translation about phosphole. |
 |
|
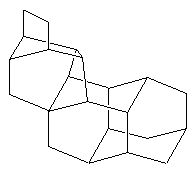
BastardaneThis is actually a close relative of adamantane, and its proper name is ethano-bridged noradamantane. However because it had the unusual ethano bridge, and was therefore a variation from the standard types of structure found in the field of hydrocarbon cage rearrangements, it came to be known as bastardane - the "unwanted child". [A. Nickon and E.F. Silversmith, 'Organic Chemistry: The Name Game', Pergamon, 1987]. |
 |
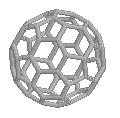   |
Buckminster FullereneThis is the famous soccerball-shaped molecule that won its discoverers
the Nobel
prize for Chemistry in 1996. It is named after the architect
Buckminster Fuller who designed the geodesic dome exhibited at Expo '67 in
Montreal, from which Sir Harry Kroto got the idea how 60 Carbon atoms
could be arranged in a perfectly symmetrical fashion. Because the name of
the molecule is a bit of a mouthful, it is often referred to just as a
Bucky
Ball. It's also known as 'Footballene' by some researchers. In
fact, there is now a whole 'fullerene
zoo', with oddly coined names, including: Buckybabies
(C32, C44, C50, C58),
Rugby Ball (C70), Giant Fullerenes
(C240, C540, C960), Russian Egg or
Bucky Onions (balls within balls), Fuzzyball
(C60H60), Bunnyball
(C60(OsO4)(4-t-Butylpyridine)2),
Platinum-Burr Ball
({[(C2H5)3P]2Pt}6C60)
and Hetero-fullerenes (in which some Cs are replaced by other
atoms). Thanks to A. Haymet for the info regarding footballene, and to Charles Turner for the names of the other fullerenes which came from: 'Fullerenes', by Robert F. Curl and Richard E. Smalley, Scientific American October 1991, and to Tom Hawkins for the JACS reference. |
MegaphoneDespite having a ridiculous name, the molecule is quite ordinary. It gets its name from being both a constituent of Aniba Megaphylla roots and a ketone. |
 |
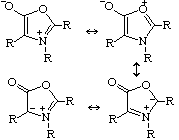 |
MunchnonesNo, these aren't the favourite compound of the Munchkins from The Wizard of Oz, but are in fact a type of mesoionic compound. These are ring structures in which the positive and negative charge are delocalised, and which cannot be represented satisfactorily by any one polar structure. They got their name when Huisgen called them after the city Munich (München), after similar compounds were called sydnones after Sydney. Huisgen et al. Chem. Ber. 1970, 103, 2611. Thanks to Matthew J. Dowd, Virginia Commonwealth University, Richmond, VA, for supplying this one. |
UnununiumI know this is technically an element, not a molecule, but it had such a ridiculous name I thought I'd include it. This is actually element number 111, and was called by the IUPAC temporary systematic name of unununium before it was recently renamed roentgenium. This is a pity, because if it formed ring or cage structures, previously we might have ended up with unununium onions... [See Pure and Appl. Chem. 51 (1979) 381 for the naming scheme]. Thanks to Chris Fellows for info about its new name. |
 |
 A sample of pyroxmangite, with white pieces of cummingtonite visible toward the lower left. |
CummingtoniteThis mineral must have the silliest name of them all! Its official name is magnesium iron silicate hydroxide, and it has the formula (Mg,Fe)7Si8O22(OH)2. It got its name from the locality where it was first found, Cummington, Massachusetts, USA. |
Putrescine, Cadaverine, Spermine and SpermidinePutrescine originates in putrefying and rotting flesh, and is quite literally, the smell of death. It is one of the breakdown products of some of the amino-acids found in animals, including humans. Although the molecule is a poisonous solid, as flesh decays the vapour pressure of the putrescine it contains becomes sufficiently large to allow its disgusting odour to be detected. It is usually accompanied by cadaverine (named after the cadavers that give rise to it), a poisonous syrupy liquid with an equally disgusting smell. Putrescine and cadaverine also contribute towards the smells of some living processes. Since they are both poisonous, the body normally excretes them in whatever way is quickest and most convenient. For example, the odour of bad breath and urine are 'enriched' by the presence of these molecules, as is the 'fishy' smell of the discharge from the female medical condition bacterial vaginosis. Putrescine and cadaverine also contribute to the distinctive smell of semen, which also contains the related molecules spermine and spermidine. Thanks to Bill Longman for suggesting spermine and spermidine, and to Dr Chris Valentine for the info about bacterial vaginosis. |
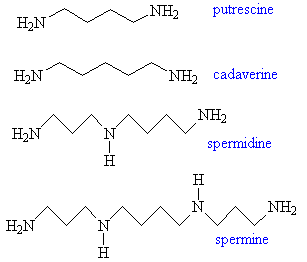
Silly names, and smelly too... |
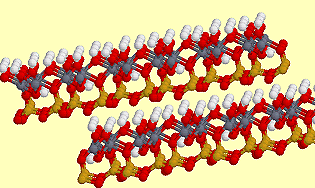
2 layers of dickite. |
DickiteDickite, Al2Si2O5(OH)4, is a (kaolin) clay-like mineral which exhibits mica-like layers with silicate sheets of 6-membered rings bonded to aluminium oxide/hydroxide layers. Dickite is used in ceramics, as paint filler, rubber, plastics and glossy paper. It got its name from the geologist that discovered it around the 1890s, Dr. W. Thomas Dick, of Lanarkshire, Scotland. Structure from the Virtual Museum of Minerals and Molecules |
Moronic AcidThis is a triterpenoid organic acid that is found in Pistacia resin, and is therefore of interest to people studying archaeological relics, shipwrecks and the contents of ancient Egyptian jars. Its name comes from its corresponding hydroxy acid, which was originally named morolic acid since it was isolated from the heartwood of the mora tree, mora excelsa. The keto acid then became moronic acid. Derivatives of this are called moronates, as in 'which moron-ate the contents of this jar?' Ref: P.L. Majumdar,
R.N. Maity, S.K. Panda, D. Mal, M.S. Raju and E. Wenkert, J. Org.
Chem. (1979) 44, 2811. |
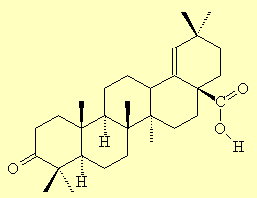
Moronic acid |
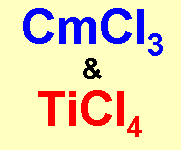 |
Curious Chloride and Titanic ChlorideThe trivial name for some curium compounds can be either curous or
'curious', so curium trichloride becomes curious chloride. However
the only curious property it has is that it's sufficiently radioactive
that a solution, if concentrated enough, will boil spontaneously after a
while. (I wonder if a molecule with 2 Cm atoms in would be
'bi-curious'...?) Thanks to Beveridge and Dr Justin E. Rigden for supplying these two and to John Burgess and Neil Tristram for the ideas on curates, and to Michael Geyer for the Nickelous content. |
FukaliteThis wonderfully named mineral gets its name from the Fuka mine in the Fuka region of southern Japan. It is very rare, and is a form of calcium silico-carbonate, with formula Ca4Si2O6(CO3)(OH,F)2. More details from: Henmi, C., Kusachi, I., Kawahara, A., and Henmi, K., Mineral. J.,8, (1977) 374. Thanks to Matthew Latto for info on this mineral. |
 |
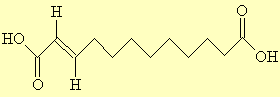
Traumatic AcidThis is a plant hormone which causes injured cells to divide and help repair the trauma - hence its name, and its synonym 'wound hormone'. Thanks to Dr Neil Edwards for supplying this one, and to Han Wermaat in the Dutch Chemistry magazine 'Chemisch2weekblad' for its information. |
 |
  |
FucitolAlthough this sounds like what an undergraduate chemist might exclaim when their synthesis goes wrong, it's actually an alcohol, whose other names are L-fuc-ol or 1-deoxy-D-galactitol. It gets its wonderful trivial name from the fact that it is derived from the sugar fucose, which comes from a seaweed found in the North Atlantic called Bladderwrack whose latin name is Fucus vesiculosis. Interestingly, there are a few articles in the Journal of Biochemistry throughout 1997 concerning a kinase enzyme which acts on fucose. The creators of these articles were Japanese, and seemed to have missed the fact that fucose kinase should not be abbreviated as 'fuc-K'. Similarly, the E. coli K-12 Gene has other proteins that have been named Fuc-U and Fuc-R. Thanks to Bob Brady for suggesting this one, and to Dr Stephen O'Hanlon from the Orthopaedics Dept of Bedford Hospital for the information on fucose kinase, and to Professor Anthony Davis of Bristol University for suggesting FucU and FucR. |
 |
KinoshitaliteAlthough it sounds like the trade name of a laxative, this is a type of mica found in Japan and Sweden, and has the formula (Ba,K)(Mg,Mn)3Si2Al2O10(OH)2. It is green and vitreous, and is about as hard as fingernails, apparently. Its name comes from the Japanese for "under the tree" (ki = tree; no = possessive particle; shita = under). Thanks to Van King for info on this mineral and Melita Rowley for the Japanese translation. |
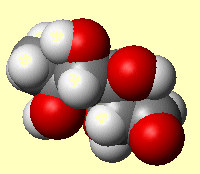 |
RhamnoseThis sounds like the molecule that's created when you walk into doors...in fact it's a type of sugar. Thanks to Bill Longman for info on this molecule. |
  |
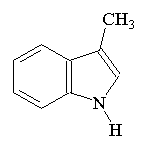 |
SkatoleThis molecule's name comes from 'skatalogical', meaning concerning fecal material. Its proper name is 3-methylindole, but it gets its trivial name from the fact that it is a component of feces. Surprisingly, it is also found in coal tar and beetroot (!), and can be obtained synthetically by mixing egg albumin and KOH. Skatole consists of white crystals when pure (or brownish scales when impure) which are soluble in hot water. Apparently, coriander can be used to cover up bad smells such as these, as testified in the classic paper: "Deodorizing Effect of Coriander on the Offensive Odor of the Porcine Large Intestine" [Kohara et al, Food Sci. Technol. Res. 12 (2006) 38.] Thanks to Allen Knutson for suggesting this molecule, and to Samuel Knight for providing the info. |
|
 |
ArsenoliteThis is a naturally occurring mineral, whose correct name is cubic arsenic trioxide (As2O3). It is also the primary product whenever arsenic ores are smelted, and is used in industry as a glass decolourising agent. Another related mineral with a similar silly name is arsenolamprite, which is a native form of arsenic. Thanks to Matthew Latto and Nicholas Welham for suggesting
these minerals. |
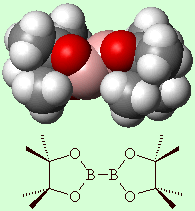 |
Bis(pinacolato)diboronAlthough it sounds like it, this isn't the active ingredient in a pina colada cocktail. Rather it is a versatile reagent for the preparation of boronic esters from halides, the diboration of olefins, and solid-phase Suzuki coupling. A proper Pina Colada cocktail is a concoction of pineapple juice, coconut milk and rum, often served with crushed ice and a little paper umbrella stuck in the glass. Thanks to Victoria Barclay of Advanced Chemistry Development, Inc., Toronto, for providing the info on this molecule. More info: Tet. Letts. 39 (1998) 2357. |
 |
 |
Lucifer yellowI think Lucifer Yellow is a food colouring used especially in hot sauces, like salsa pickle. It is also used in plant microscopy anatomy studies, because it fluoresces under ultraviolet light and stains certain regions between plant cells. For more info, see here. Thanks to Gavin Shear of Advanced Chemistry Development, Inc., Toronto, and to Seranne Howis, of Rhodes University, South Africa, for providing the info on this molecule. |
 |
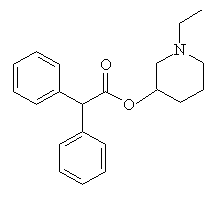
CrapinonCrapinon (also known as Sanzen) is another molecule with an excellent name, and is apparently used therapeutically as an anticholinergic. These are drugs which dry secretions, increase heart rate, and decrease lung constriction. More importantly, they also constipate quite strongly - so 'crappy-non' is most appropriate. It would be nice to think that this molecule could find an alternative use as a toilet cleaner (as in "Who's been crapinon the seat?"). Thanks to Gavin Shear of Advanced Chemistry Development, Inc., Toronto, and to Tom Simpson of the Royal Hobart Hospital, Austalia for providing the info on this molecule. |
 |
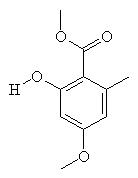 |
SparassolThis molecule sounds like what you'd need the day after eating a very hot curry (spare-assol). Sparassol is an antibiotic produced by the fungus Sparassis ramosa. Thanks to Eric Walters from The Chicago Medical School for providing the info on this molecule. |
Periodic AcidOk, I know it should really be per-iodic acid, but without the hyphen it sounds like it only works some of the time...It has also been described as that acid extracted by boiling of old periodic tables found in chemistry lecture halls and laboratories. Thanks to Allen Knutson for suggesting this molecule, and to Prof Walter Maya of California State Polytechnic University for some of the details. |
 |
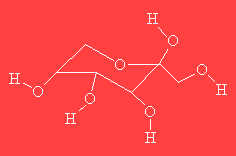 |
PsicoseThis molecule has nothing to do with axe-murderers, but is a sugar which gets its name because it's isolated from the antibiotic psicofurania. Its other name is ribo-hexulose. |
 |
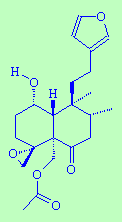 |
FruticoloneThis sounds like what you get after a baked bean meal...but it actually gets its name from being both a constituent of the plant Teucrium fruiticans and a ketone. |
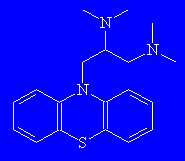 |
SpamolMonty Python's favourite molecule? Spamol might also conjure images of unwanted "Make Money Fast" emails circulating the globe at the speed of light ("spam - all"). Its other names are aminopromazine, lispamol or lorusil, and it's actually used as an anti-spasmodic therapeutic agent. Thanks to Victoria Barclay of Advanced Chemistry Development, Inc., Toronto, for providing the info on this molecule. |
 |
FuniconeThis gets its name, not from being funny and cone shaped, but because it's the metabolism product of the fungus Penicillium funiculosum. |
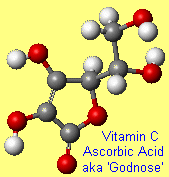
GodnoseOk, so this is a bit of a cheat, since it's not an official molecular name...but it makes a nice story. When Albert Szent-Gyorgyi isolated ascorbic acid and published his findings, he called the new substance 'ignose' since he was convinced it was a sugar that resembled glucose and fructose, but was ignorant of its structure. When the journal editor refused to accept ignose as a sensible name, Szent-Gyorgyi suggested 'Godnose' instead! Alas the editor was neither imaginative nor humorous, and suggested that a more proper name had to be used. The structure of the carbohydrate was elucidated in collaboration with Haworth at Birmingham and the alternative name given was hexuronic acid (hex = six). During the same period (1928–1931), Charles Glen King of the Columbia University of USA isolated Vitamin C from lemon juice and it was observed that hexuronic acid and Vitamin C were identical. Szent-Györgyi documents the episode in the essay "Lost in the Twentieth Century" which dates from 1963. Thanks to John P Oliver, Peter Macinnis and Charles Turner for providing the info and story. Incidentally, Godnose is also the name of an Australian punk band. |
 |
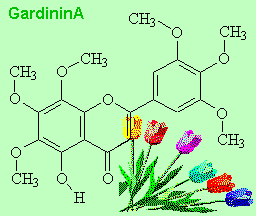 |
Gardenin
|
GermaneThis is a particularly relevant molecule that is pertinent and has a bearing on a number of inorganic reactions... Thanks to Eric Walters from The Chicago Medical School for suggesting this molecule. |
 |
Uranate |
  |
The various uranium oxide anions (UO22-,
UO32-, UO42-, etc) go by the
glorious name of 'uranates'. I wonder if unwanted reactions of these ions with
certain compounds is called 'involuntary uranation'...? And is nickel uranate
what you'd need to 'spend a penny'?
Related to this, uranium nitrate is also
known as uranyl nitrate, which sounds like the entry fee for a gents toilet
after 8pm... While U4+ is the uranous ion. This sparks the question
that when U4+ is in aqueous solution, do the water molecules form a
ring around uranous? An if you do need to uranate, should you do it at the
tungsten carbide (WC)?
Thanks to Victor Sussman from Carleton College in Northfield, MN, USA for suggesting this molecule, to Amy Roediger for suggesting nickel uranate, to Sunil Rao for uranous and to Andy Shipway for WC.
 |
Kunzite (Spodumene)This mineral is a pink (of course...) gemstone, named after the gemologist G.F. Kunz who described it in 1902. Kunzite is a fragile stone, which shows different shades of colour when viewed from different directions. Called an "evening" stone, it should not be exposed to direct sunlight which can fade its color in time. Its alternative name, Spodumene, sounds like an American shop that sells computer nerds ("Spod-U-Mean") |
 |
WelshiteThis wonderfully named mineral is called after the US amateur mineralogist Wilfred R. Welsh. Its formula is Ca2SbMg4FeBe2Si4O20. Some people think it's quite a nice mineral, but others think it's 'well-shite'. Thanks to Matthew Latto for suggesting this mineral. |
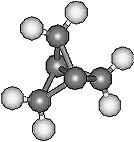 |
Propellane and CubaneThese two molecules are both named after their distinctive shapes. Propellane (left), C5H6, resembles a propeller, whereas cubane (right), C8H8, is a cube (but doesn't come from Cuba). Other molecules that get their name from their geometric shapes are: dodecahedrane C20H20, prismane C6H6, spherands and hemispherands, squaric acid C4H2O4 and deltic acid C3H2O3, tetrahedranes C4H4 and C20H36 and finally twistane C10H16. Thanks to Kay Brower for suggesting propellane, and to Martin A. Iglesias Arteaga from the University of Havana, Cuba for suggesting cubane. Thanks also to Mark Minton for suggesting the other list of geometrical molecules. First reference for propellane: J. Altman , E. Babad, J. Itzchaki, D. Ginsburg , Tetrahedron, Suppl. 8(1), (1966) 279. |
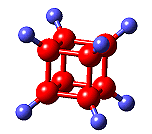 |
ClitoriacetalThis gets its name from the root of the Clitoria macrophylla plant, and is a constituent of the Thai drug "Non-tai-yak" which is used to treat respiratory disorders, including pulmonary tuberculosis and bronchitis, and also works as an insecticide. |
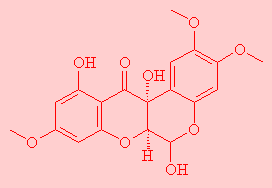 |
 |
BuccalinThis sounds like the molecule from which car seat-belts are made, but it's actually a neuropeptide which acts in nerves to stop acetylcholine release. Thanks to Dr. Andrew P. Rodenhiser from McGill University, Canada, for suggesting this molecule. |
FornaciteThis is a mineral that is composed of a basic chromate-arsenate compound of Pb and Cu with the formula: (Pb,Cu2+)3[(Cr,As)O4]2(OH). If it could be polished into a gemstone, it sounds ideal for a ring that a cheating husband might buy his mistress. Thanks to Alan Plante, a New Hampshire, USA, "Rockhound", for suggesting this mineral. |

The dark lumpy bits on the white background are Fornacite |
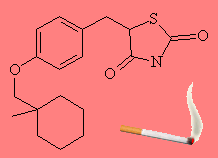
CiglitizoneThis molecule sounds like the places reserved for smokers to light up. Actually, ciglitizone is is a member of a class of compounds that are used as anti-diabetics. The drug Avandia (Rosiglitazone), used to treat type II diabetes, is a member of this class of compounds. Another related molecule is troglitazone, which I've mentioned for all fans of the eponymous rock group or small cave dwelling dwarfs. Thanks to Robin Brown of Galapagos Genomics, Belgium, and to Joerg Fruechtel for the info on this molecule. (More info, see: Br. J. Pharmacol. 131 (2000) 651). |
 |
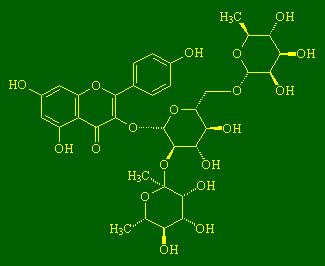 |
ClitorinI don't know much about clitorin, except that it's a flavenol glycoside (make of that what you will), but I've heard it's touch sensitive ;-). Thanks to Joerg Fruechtel and Nicholas J. Welham for the info on this molecule. (More info, see: Chem. Pharm. Bull. 53 (2005) 591). |
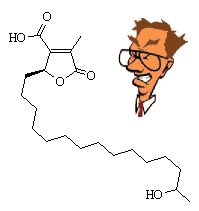
Constipatic AcidThis is a constituent of some Australian lichens, such as Parmelia constipata. I don't know why these lichens were called this, maybe eating them causes constipation? Derivatives of constipatic acid include protoconstipatic acid, dehydroconstipatic acid, and methyl constipatate. See D.O. Chester and J.A. Elix, Austr. J. Chem. 32 (1979) 2565. Thanks to Ronald Wysocki of the University of Arizona for suggesting this molecule. |
 |
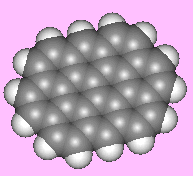 |
OvaleneOvalene is a polycyclic aromatic hydrocarbon, C32H14. Funnily enough it's oval-shaped... In the series of polycyclic aromatic hydrocarbons to which ovalene belongs, the next one is circumanthracene...(is it also known as oy-vane?). Thanks to Terry Frankcomb of the University of Queensland, Australia for suggesting this molecule, and to Professor Juan Murgich of the Venezuelan Institute for Scientific Research, Caracas, for providing a correct structure. |
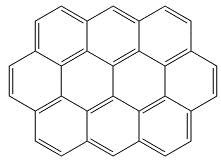 |
AenigmatiteThis mineral gets its 'enigmatic' name from the fact that its chemical composition was originally difficult to determine. It is an iron and titanium silicate with sodium as a charge balancing cation, although because it does not easily fit into the current classification system, it is classified as an 'Unclassified Silicate'. Thanks to Phillip Barak of the Virtual Museum of Minerals and Molecules for suggesting this mineral. |
 |
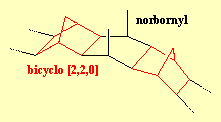  |
DogcollaraneDogcollaranes are a group of molecules made from alternating bicyclo [2,2,0] and norbornyl segments. When there are 24 such components, the ends can be linked together to form a ring, which looks like a dogcollar. Unfortunately, although many of the intermediate structures have been made, none of the dogcollaranes have yet been synthesised. For more info, see: Aust. J.
Chem. 40 (1987) 1951. |
FuchsiteFuchsite is a mineral, and is the green form of Muscovite, KAl2(AlSi3O10)(F, OH)2. It is used as an ornamental stone, and apparently has perfect cleavage... Thanks to Tanuki for suggesting this mineral. |
 |
 |
PagodaneThis C20H20 molecule gets its name because it resembles a Japanese pagoda - well, two pagodas, back-to-back. Thanks to Mark Minton for suggesting this molecule. |
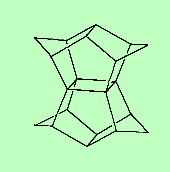 |
 |
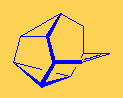
SnouteneThis strange looking molecule resembles the nose or snout of an animal, but I don't know if it smells... Thanks to Mark Minton for suggesting this molecule. |
 |
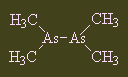 |
CacodylThis molecule gets its name from the Greek kakodes, meaning 'stinking', as it has a really pungent smell of manure with a delicate hint of garlic. It is sometimes spelled 'kakodyl', but its correct name is tetramethyl diarsenic. Its main claim to fame is that it was one of the compounds worked on by Bunsen (of burner fame). Thanks to Birgit Schulz for suggesting this molecule and to Lars Finsen for correcting the spelling of the name. |
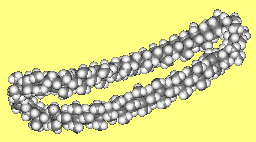
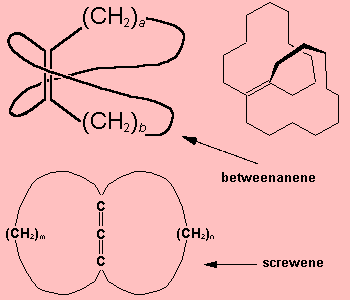
Betweenanene (Screwene)Betweenanenes are molecules which have a trans double bond shared between two cycloalkanes. There is a whole family of them, depending upon the size of each ring. The one shown on the right is the [10,10] betweenanene. If there are two double bonds linked together, the molecules are called screwenes, but this terminology isn't that popular, for obvious reasons! More info see: J. Am. Chem. Soc. 99 (1977) 3508. Thanks to Mark Minton for suggesting this molecule. |
 |
PaddlanePaddlanes are molecules which have bicyclic cyclohexane units, which look a bit like the paddles on Mississippi steamboats. Thanks to Mark Minton for suggesting this molecule. |
 |
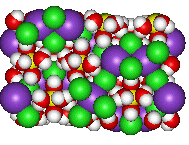
CarnalliteCarnallite is KMgCl3·6H2O, an evaporite mineral. Surprisingly for a mineral called carnallite, it doesn't exhibit any cleavage... It's used as an ore for potassium fertilizers, and is named after Rudolf von Carnall, a Prussian mining engineer, whose (Carnall?) knowledge of the subject was famous... Thanks to Phillip Barak of the Virtual Museum of Minerals and Molecules for suggesting this mineral. |
 |
 |
DraculinDraculin is the anticoagulant factor in vampire bat saliva. It is a large glycoprotein made from a sequence of 411 amino acids. Thanks to Mark Baxter for suggesting this molecule, to Eric Walters for some of the info about it and to Tara Turnas for the molecular structure image. |
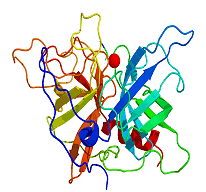 |
![]() You're visitor no.
You're visitor no.  .
Last modified Wednesday, 28-Oct-2009 09:40:26 GMT. Paul
May.
.
Last modified Wednesday, 28-Oct-2009 09:40:26 GMT. Paul
May.